Chemical name:
Synthetic Sodium Hydrated Aluminum Silicate
Chemical structure:
SOFONITE 2 in 1 is a kind of sodium bentonite made from processing calcium bentonite. Bentonites are three-layer minerals consist of two tetrahedral layers sandwiched around a central octahedral layer. Anionic oxides in the lower side of the upper layer and the upper side of the lower layer through connection with the cation of aluminum, magnesium or iron in the middle level create the octahedral structure. The connection between shared internal anionic oxides and cations is the reason for connection of layers, creating the integrated bentonite structure.
In the structure of bentonite, the sum of negative charges of the anionic oxides are a bit more than the sum of the cationic positive charges of the structure. On the other hand, the surface of the bentonite sheets is made with anionic oxides and the cations are placed between the sheets. These two reasons cause the negative charge on the surface of the bentonite sheets. This negative charge on the surface reaches its balance through attracting free cations placed between the bentonite sheets.
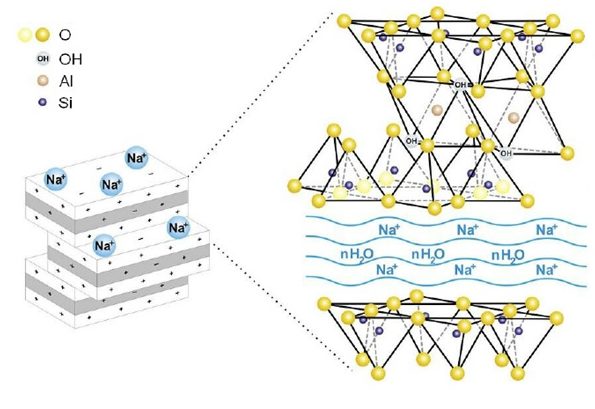
Each individual bentonite platelets that consist three layers, are about 1 nanometer thick and between 0.2 to 2 microns wide. These sheets in dry state usually place on each other in parallel. In this condition, a little adsorbed water and free cations are between platelets; therefore, the thickness of the interlayer region is variable depending on the amount of water adsorbed between the platelets.
Generally, bentonites are classified based on their dominant exchangeable interlayer cation. For example, if among the exchangeable cations between the sheets such as sodium, calcium and magnesium, the amount of sodium cation is more than calcium or magnesium ones, the bentonite is called sodium bentonite. Sodium bentonite attracts a lot of water between its sheets. Therefore, its swelling volume is more significant than the others.

